Completion of delayed cases in the licensing area
The Danish Medicines Agency publishes updates on the status of delayed cases in the licensing area every second month in mid-June, mid-August, mid-October and mid-December 2023.
Throughout the years of the COVID-19 pandemic, the Danish Medicines Agency has built up a backlog of cases that fail to meet the review times established in the licensing area. Extra resources have been allocated to the area, and we expect to have completed the delayed cases by the end of 2023. We will complete the cases gradually over the year, so the number of delayed cases will decline, the more we advance into the year.
The charts below show the position of the Danish Medicines Agency in relation to the established objectives for handling. To succeed, it is assumed that the regular intake of new cases remains at the normal level. Throughout the year, we might see fluctuations in the number of new cases we take on, and, as a result, we might deviate periodically from the completion plan. We usually receive an increased number of cases before summer and at the turn of the year. Likewise, some of the case type areas necessitate particularly experienced employees, requiring us to allocate these employees to focused projects throughout the year. And that might also cause us to deviate from the completion plan.
We will be publishing charts for the following case types:
- National variations, type IB and type II (only delayed cases)
- RMS variations, type IB and type II (all cases)
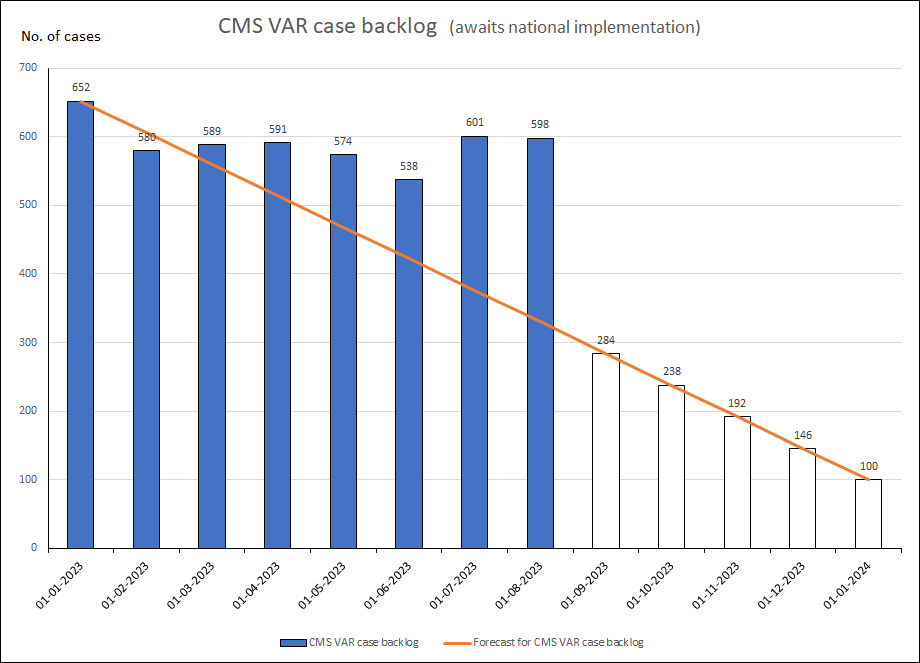
- CMS variations (closed procedures)
- Parallel import applications
In addition, we will show the progress of cases in the order books that are published on our website.
Update on the completion of delayed cases in the licensing area as of end-July 2023
As of end-July 2023, we make the following observations:
The Danish Medicines agency usually has an increase in delayed cases during the summer holidays. This is due to a high amount of received applications before the start of the summer holidays. In the period after the summer holiday months, this corrects itself. This can be seen in the graphs below.
Concerning national and RMS type IB variations: Due to the summer holidays, few cases have been initiated and this can be seen on the graph, but it is still expected that we will reach the target for the whole year.
Concerning national and RMS type II variations: We have continued to receive an extraordinary number of RMS applications in June and July and this is reflected in an increased number of delayed cases in relation to the set target. For both types of variations, we will have extra focus in the coming months.
Concerning completion of CMS variations: The graph shows an increase in delayed cases, this is due to summer holidays. It is expected that the amount of delayed case will be reduced in the months after the summer holidays.
Concerning Parallel import applications: The Danish Medicines Agency has of end-May 2023 met the set target for these applications. We will therefore no longer publish this graph, as we continue to work on completing the remaining delayed cases and at the same time for new applications will work according to the set target for assessment time.
Update as of end-July 2023
Update on the completion of delayed cases in the licensing area as of end-May 2023
As of end-May 2023, we make the following observations:
Concerning national and RMS type IB variations: The Danish Medicines Agency is slightly behind in relation to the set target, but it is expected that we will reach the target for the whole year.
Concerning national and RMS type II variations: we received an extraordinary number of RMS applications in May and this is reflected in an increased number of delayed cases compared to the set target. For both types of variations, we will have extra focus in the coming months.
Concerning completion of CMS variations: We are somewhat behind the set target, but still maintain a downward curve. Experienced employees. We expect to be back on track after the summer vacation.
Concerning Parallel import applications: The Danish Medicines Agency har met the set target for these cases.
Update as of end-May 2023
Update on the completion of delayed cases in the licensing area as of end-March 2023
The Danish Medicines Agency publishes updates on the status of delayed cases in the licensing area every second month in mid-June, mid-August, mid-October and mid-December 2023. To begin with, the results for Q1 2023 are shown below.
Throughout the years of the COVID-19 pandemic, the Danish Medicines Agency has built up a backlog of cases that fail to meet the review times established in the licensing area. Extra resources have been allocated to the area, and we expect to have completed the delayed cases by the end of 2023. We will complete the cases gradually over the year, so the number of delayed cases will decline, the more we advance into the year.
The charts below show the position of the Danish Medicines Agency in relation to the established objectives for handling. To succeed, it is assumed that the regular intake of new cases remains at the normal level. Throughout the year, we might see fluctuations in the number of new cases we take on, and, as a result, we might deviate periodically from the completion plan. We usually receive an increased number of cases before summer and at the turn of the year. Likewise, some of the case type areas necessitate particularly experienced employees, requiring us to allocate these employees to focused projects throughout the year. And that might also cause us to deviate from the completion plan.
We will be publishing charts for the following case types:
- National variations, type IB and type II (only delayed cases)
- RMS variations, type IB and type II (all cases)
- CMS variations (closed procedures)
- Parallel import applications
In addition, we will show the progress of cases in the order books that are published on our website.